On March 31, 2022, Silo Pharma (OTCQB: SILO) announced an agreement with contract research organization Frontage Laboratories for an Investigational New Drug (“IND”)-enabling, “Pharmacokinetic Study.” Silo merges traditional therapeutics with psychedelic research to relieve patients suffering from PTSD, Alzheimer’s, Parkinson’s, and other rare neurological disorders. Frontage will study Silo’s Central Nervous System Peptide, SPU-16, “a potential new treatment for multiple sclerosis and other conditions,” and their Joint Homing Peptide, SPU-21, used for treating arthritogenic processes, which, according to Silo Pharma CEO Eric Weisblum, could, “enhance the therapeutic effect of current and future therapeutics while decreasing potential systemic toxicity….They may be used to treat both Central Nervous System and Autoimmune Diseases.”
Pharmacodynamics studies the reactions between drugs and organisms or, simply, the effect of a drug on the body, allowing for a patient’s existing diseases or conditions, their age and other drugs they’re currently taking. Conversely, pharmacokinetics studies the body’s effect on a drug, its “absorption, distribution, metabolism, and excretion,” or the ways and rates a therapeutic enters, courses through, is modified by and finally exits the system.
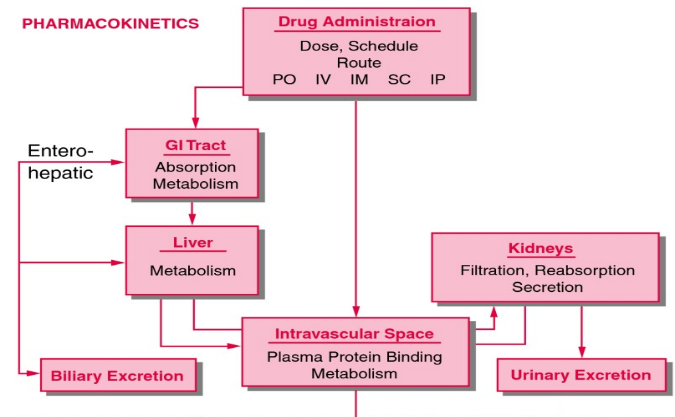
From: Principles of Pharmacokinetics, Copyright © 2003, BC Decker Inc.
DMPK (drug metabolism and pharmacokinetics) constitutes a “core discipline in drug development” for assessing drug safety. According to a Frontage Labs factsheet, DMPK studies provide critical research data, assisting therapeutics innovators like Silo to reach important milestones and “decision making during drug discovery and development.” Weisblum says the agreement (with Frontage), “significantly advances our Central Nervous System Peptide, SPU-16, and our Joint Homing Peptide, SPU-21 technologies closer to the clinic.”
For a closer look at Pharmacokinetics, check out: The National Library of Medicine, Technology Networks: Drug Discovery, the XenoTech Blog and the Frontage Resource Library.
Silo Pharma, headquartered in Englewood Cliffs, New Jersey, identifies assets to license and also funds research to better serve patients and advance the health care industry. Recent achievements include a research agreement with Columbia University developing psychedelic therapeutics for Alzheimer’s disease, DEA approval advancing (with Zylo Therapeutics) Z-Pod technology for delivering time-released Ketamine or Psilocybin, a patent for Silo’s Central Nervous System Homing Peptide, granted by the United States Patent and Trademark Office and a sponsored research agreement with UMB evaluating the pharmacokinetics of dexamethasone delivered to arthritic rats via liposomes.
For Silo Pharma’s most recent news, click here.
Silo Pharma
560 Sylvan Avenue, Suite 3160
Englewood Cliffs, NJ 07632
For more information, visit the company’s website at www.SiloPharma.com.
NOTE TO INVESTORS: The latest news and updates relating to SILO are available in the company’s newsroom at https://ibn.fm/SILO
About InvestorWire
InvestorWire is the wire service that gives you more. From regional releases to global announcements presented in multiple languages, we offer the wire-grade dissemination products you’ll need to ensure that your next press release grabs the attention of your target audience and doesn’t let go. While our competitors look to nickel and dime you with hidden fees and restrictive word limits, InvestorWire keeps things transparent. We offer UNLIMITED Words on all domestic releases. While other wire services may provide a basic review of your release, InvestorWire helps you put your best foot forward with complimentary Press Release Enhancement.
With our competitors, the work is done the second your release crosses the wire. Not with InvestorWire. We include follow-up coverage of every release by leveraging the ever-expanding audiences of the 50+ brands that make up the InvestorBrandNetwork.
Get more out of your next press release with InvestorWire. It’s unlike anything you’ve seen before.
For more information, please visit https://www.investorwire.com
Please see full terms of use and disclaimers on the InvestorBrandNetwork website applicable to all content provided by IBN, wherever published or re-published: http://ibn.fm/Disclaimer
InvestorWire (IW)
8033 Sunset Blvd Suite 1037-IW
Los Angeles, CA 90046
310.299.1717 Office
www.investorwire.com
[email protected]
InvestorWire is part of the InvestorBrandNetwork.
